N2 MEMBER PORTAL
N2’s tools, resources and educational materials are available to N2 members. We currently hold our N2 resources behind a password-protected site. If you do not know your login information, please get in touch with N2 for assistance. What resources are available with your N2 membership? Learn more here.
The Canadian Clinical Trials Asset Map
Access the Canadian Clinical Trials Assets Map hosted by N2 Canada
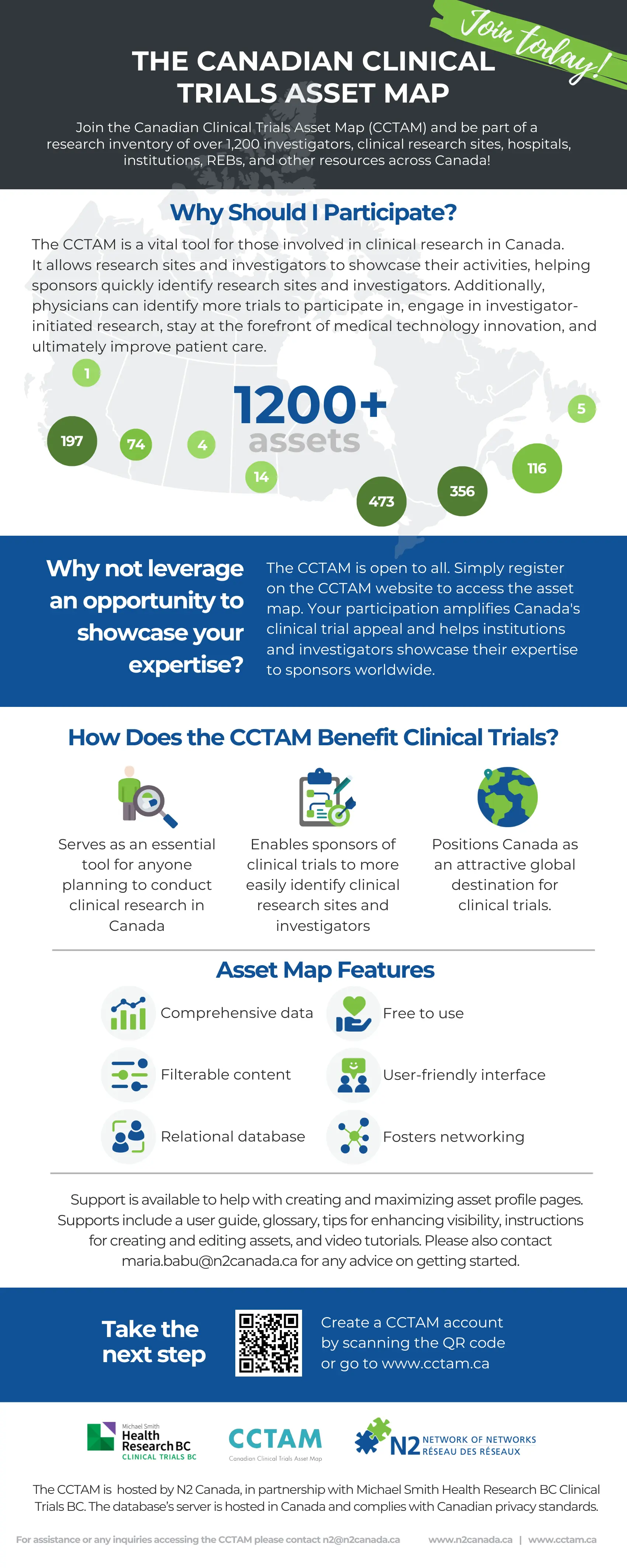
The Canadian Clinical Trials Asset Map (CCTAM) is a pan-Canadian research inventory of investigators, clinical research sites, hospitals, institutions, research ethics boards (REBs) and other clinical research resources available across the country. The CCTAM was developed to position Canada as a premier destination for clinical trials by creating an extensive database of Canadian clinical research assets.
Read More
The Asset Map is an essential tool for anyone considering or planning to conduct clinical research in Canada. It is an ideal way for sponsors of clinical trials to identify both suitable clinical research sites and investigators that can help expedite study-feasibility analysis and advance research activities. It is also a tool for physicians who want the opportunity to participate in more trials, engage in investigator-initiated research and, above all, stay at the forefront of innovation and medical technology to ultimately provide better care for their patients.
The Asset Map places Canada in a strong position to attract clinical trials and provides research institutions and investigators with the opportunity to showcase their expertise to clinical trial sponsors in Canada and around the world.
Hosted in Canada in full compliance with Canadian privacy laws and standards, this comprehensive and searchable database now includes more than 1,00 assets and it continues to grow.
The asset map was created in 2015 with input from many clinical trials thought leaders across Canada including Clinical Trials BC, CATALIS Québec, Innovative Medicines Canada, HealthCareCAN, Canadian Institutes of Health Research and Health Charities Coalition of Canada, as members and funding partners of the Canadian Clinical Trials Coordinating Centre (CCTCC). The CCTCC provided funding and engagement until 2022. Clinical Trials BC, part of Michael Smith Health Research BC, hosted and managed the CCTAM until early 2024, when N2 became the host in partnership.
What the CCTAM does for Clinical Trials in Canada:
- Enables sponsors of clinical trials to more easily identify clinical research sites and investigators
- Serves as an essential tool for anyone planning to conduct clinical research in Canada
- Positions Canada as an attractive global destination for clinical trials.