Tools to Support Study Planning
A strong research plan lays the foundation for a successful clinical trial. From identifying investigators and sites to supporting participant recruitment and engagement, these tools are designed to help streamline the planning process and improve study feasibility. Whether you’re launching a new trial or expanding an existing program, these resources can help you connect, prepare, and move forward with confidence.
This section includes tools, registries, and resources to help you:
-
Access a national database of clinical trial assets across Canada
-
Use searchable registries to explore active and past clinical trials
-
Support participant recruitment with tools for engagement strategies
-
Strengthen study feasibility and enhance site selection
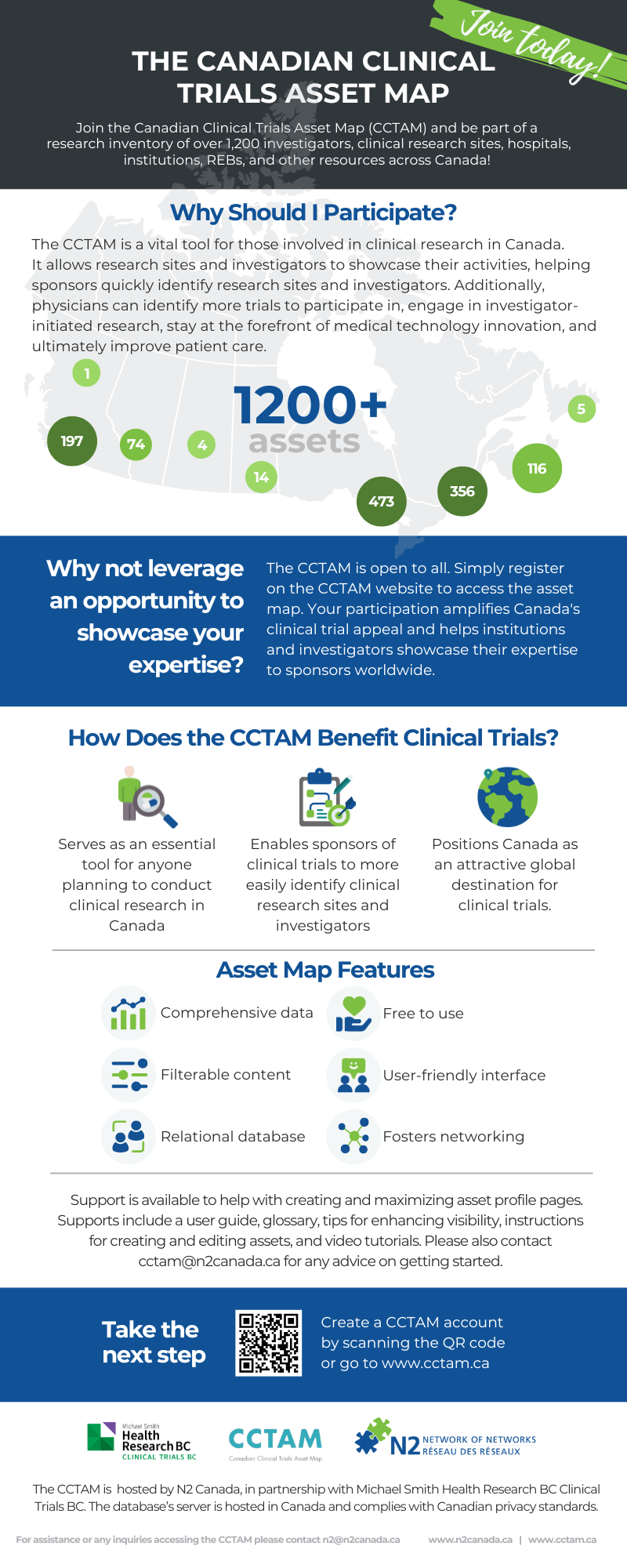
The Canadian Clinical Trials Asset Map (CCTAM)
Available in English | Open Source
The Canadian Clinical Trials Asset Map (CCTAM) is a national database of investigators, sites , and research infrastructure across Canada. It supports study planning and feasibility by helping sponsors and researchers identify collaborators quickly and confidently.
The Canadian Clinical Trials Asset Map (CCTAM) is a pan-Canadian inventory of clinical research assets, including investigators, research sites, hospitals, institutions, and research ethics boards (REBs). It offers a clear view of the clinical research landscape across Canada.
Designed to support study planning and feasibility, the Asset Map is a valuable tool for anyone looking to conduct research in Canada. Sponsors can quickly identify qualified investigators and research sites, while physicians can explore opportunities to participate in trials, lead investigator-initiated studies, and remain engaged with the latest medical innovations.
By showcasing Canada’s clinical research capacity, the CCTAM strengthens the country’s global position as a leading destination for clinical trials. It also helps research institutions and investigators demonstrate their expertise to sponsors both nationally and internationally.
Fully hosted in Canada and compliant with Canadian privacy laws and standards, the searchable database now includes over 1,100 assets—and continues to grow. The CCTAM is managed by N2 Canada, in partnership with Clinical Trials BC, and was originally developed in 2015 by the Canadian Clinical Trials Coordinating Centre with support from Clinical Trials BC, CATALIS Québec, Innovative Medicines Canada, HealthCareCAN, and the CIHR Strategy for Patient-Oriented Research (SPOR).
The CCTAM helps to:
-
Support site and investigator identification for clinical trial sponsors
-
Provide a trusted resource for study feasibility and planning
-
Position Canada as a globally competitive destination for clinical research
Permission to Contact (PTC) Toolkit
Available in English | Member Access Only
Designed to support inclusive enrollment
The Permission to Contact (PTC) platform is a participant enrollment strategy designed to support inclusive and efficient recruitment for clinical research. By integrating a simple question—“Would you like to be contacted for future research opportunities?”—into routine clinical care, the platform helps overcome one of the biggest barriers to research participation.
PTC Table of Contents
Foreword
Acknowledgements
Abbreviations and Acronyms
1. PTC Toolkit – Planning Phase
1.1 Strategic Checklist
1.2 Budget Considerations
1.3 Governance Framework
2. PTC Toolkit – Stakeholder Engagement Phase
2.1 Stakeholder Engagement Plan
2.2 Communications Strategy
2.3 Privacy FAQs
2.4 Research Ethics FAQs
2.5 General Operations FAQs
3. PTC Toolkit – Development Phase
3.1 Protocol Template
3.2 Data Management
3.3 Participant Materials
4. PTC Toolkit – Implementation Phase
4.1 Staff Training Materials
4.2 Research Evaluation
5. Monitoring and Evaluation
6. Other Resources Available
6.1 The Biobank Resource Centre
6.2 Useful Websites
6.3 Contact
6.4 Glossary
6.5 References
Appendices
-
Appendix A – Survey Approach and Guidelines
-
Appendix B – Example Surveys
-
Appendix C – Stakeholder Survey Table
-
Appendix D – Documenting Permissions SOP Template
-
Appendix E – Example PTC Participant SOP Template
-
Appendix F – Example PTC Process Map
-
Appendix G – Example PTC Database Application Fields and Definitions
-
Appendix H – Examples of Participant Materials
-
Appendix I – Example Research Application Form
-
Appendix J – Example Telephone Script When Contacting PTC Participants
What’s Included?
The PTC Toolkit offers clear, practical guidance for implementing the platform in your organization. It covers budgeting, stakeholder engagement, data planning, and readiness steps. Created with OBER, Island Health, and CTRNet, it supports national adoption of core PTC practices.
Available to members of N2 or the Biobank Resource Centre, the toolkit also includes optional consulting support—like project management, training, SOP development, and ethics application guidance. English only. Member access required.
Clinical Trial Registries
Looking for information about clinical trials in Canada or around the world? These registries offer reliable, searchable access to ongoing and completed studies. Whether you’re a researcher, health care provider, or member of the public, these tools support transparency, planning, and informed decision-making.
Canadian Cancer Trials
A national platform focused on cancer clinical trials in Canada. Search by cancer type, location, or trial phase to find opportunities for participation or collaboration.
– Visit Canadian Cancer Trials
ClinicalTrials.gov
A global registry of federally and privately supported clinical trials. Search for studies by condition, location, and sponsor. Includes details on trial purpose, eligibility, and contacts. Use in consultation with health care providers.
Health Canada Clinical Trials Database
A Canadian government database listing Phase I–III clinical trials conducted with patients. Supports transparency in Health Canada-regulated research.
– Visit Health Canada’s Clinical Trials Database
ISRCTN Registry
The International Standard Registered Clinical/soCial sTudy Number (ISRCTN) registry includes trials that are proposed, ongoing, or completed, across a wide range of therapeutic areas.
WHO ICTRP
The World Health Organization’s International Clinical Trials Registry Platform (ICTRP) aggregates global trial data from multiple national and regional registries.